Introduction to DESeq2¶
This notebook serves as a tutorial for using the DESeq2 package. Please be sure to consult the excellent vignette provided by the DESeq2 package. Hopefully, we will also get a chance to review the edgeR package (which also has a very nice vignette which I suggest that you review)
Load packages¶
Load requisite R packages.
In [1]:
library(DESeq2)
library(tools)
library(limma)
library(qvalue)
library(dplyr)
options(width=100)
Loading required package: S4Vectors
Loading required package: stats4
Loading required package: BiocGenerics
Loading required package: parallel
Attaching package: ‘BiocGenerics’
The following objects are masked from ‘package:parallel’:
clusterApply, clusterApplyLB, clusterCall, clusterEvalQ,
clusterExport, clusterMap, parApply, parCapply, parLapply,
parLapplyLB, parRapply, parSapply, parSapplyLB
The following objects are masked from ‘package:stats’:
IQR, mad, xtabs
The following objects are masked from ‘package:base’:
anyDuplicated, append, as.data.frame, cbind, colnames, do.call,
duplicated, eval, evalq, Filter, Find, get, grep, grepl, intersect,
is.unsorted, lapply, lengths, Map, mapply, match, mget, order,
paste, pmax, pmax.int, pmin, pmin.int, Position, rank, rbind,
Reduce, rownames, sapply, setdiff, sort, table, tapply, union,
unique, unsplit, which, which.max, which.min
Attaching package: ‘S4Vectors’
The following objects are masked from ‘package:base’:
colMeans, colSums, expand.grid, rowMeans, rowSums
Loading required package: IRanges
Loading required package: GenomicRanges
Loading required package: GenomeInfoDb
Loading required package: SummarizedExperiment
Loading required package: Biobase
Welcome to Bioconductor
Vignettes contain introductory material; view with
'browseVignettes()'. To cite Bioconductor, see
'citation("Biobase")', and for packages 'citation("pkgname")'.
Attaching package: ‘limma’
The following object is masked from ‘package:DESeq2’:
plotMA
The following object is masked from ‘package:BiocGenerics’:
plotMA
Attaching package: ‘dplyr’
The following object is masked from ‘package:Biobase’:
combine
The following objects are masked from ‘package:GenomicRanges’:
intersect, setdiff, union
The following object is masked from ‘package:GenomeInfoDb’:
intersect
The following objects are masked from ‘package:IRanges’:
collapse, desc, intersect, regroup, setdiff, slice, union
The following objects are masked from ‘package:S4Vectors’:
first, intersect, rename, setdiff, setequal, union
The following objects are masked from ‘package:BiocGenerics’:
combine, intersect, setdiff, union
The following objects are masked from ‘package:stats’:
filter, lag
The following objects are masked from ‘package:base’:
intersect, setdiff, setequal, union
Import Data¶
The first step to any analysis is to import the data into an analysis ready format. The latter depends on the requirements of the package used for the analysis.
For this analysis, we will use the DESeq2::DESeqDataSetFromHTSeqCount. This function allows you to import count files generated by HTSeq directly into R. If you use a program other than HTSeq, you should use the DESeq2::DESeqDataSetFromMatrix function.
Let’s review the three main arguments of DESeq2::DESeqDataSetFromHTSeqCount: sampleTable, directory and design
In [2]:
#? DESeqDataSetFromHTSeqCount
First set the directory under which the HTSeq count files are stored
In [3]:
datadir<-"/home/jovyan/work/2017-HTS-materials/Materials/Statistics/08032017/Data/2015"
Next, put the filenames into a data frame
In [4]:
phdata<-data.frame(fname=list.files(path=datadir,pattern="*.csv"),stringsAsFactors=FALSE)
head(phdata)
| fname | |
|---|---|
| 1 | 7A_E.csv |
| 2 | 7A_G.csv |
| 3 | 7A_K.csv |
| 4 | 7A_N.csv |
| 5 | 7A_P.csv |
| 6 | 7B_E.csv |
It is always a good idea to check the dimension of the file you have read in
In [5]:
dim(phdata)
- 30
- 1
Extract the label from the filename. Why should one reorder columns? See DESeq2 help file
In [6]:
phdata <- phdata %>% transmute(sample=substr(fname,1,4),fname)
head(phdata)
| sample | fname | |
|---|---|---|
| 1 | 7A_E | 7A_E.csv |
| 2 | 7A_G | 7A_G.csv |
| 3 | 7A_K | 7A_K.csv |
| 4 | 7A_N | 7A_N.csv |
| 5 | 7A_P | 7A_P.csv |
| 6 | 7B_E | 7B_E.csv |
For any analysis, you should have a manifest file that maps the file names (containing the counts) to the phenotypic and experimental condition for the sample. This file should be validated and put under strict vesrion control.
For this demonstration, we will reproduce this file by parsing the file names. This is meant to help us practice R programming.
Add some design info to the data frame. We will add the treatment factor (the first character of the file name: 7 or 8), the replicate id (the second character of the file name)
Note that tools::md5sum will add the MD5 signature for each of the HTSeq count files. You should keep track of these for the purpose of conducting reproducible analysis
In [7]:
phdata <- phdata %>% mutate(trt=as.factor(substr(sample,1,1)),
repl=substr(sample,2,2),
team=substr(sample,4,4),
md5=tools::md5sum(file.path(datadir,fname)))
head(phdata)
| sample | fname | trt | repl | team | md5 | |
|---|---|---|---|---|---|---|
| 1 | 7A_E | 7A_E.csv | 7 | A | E | 263e8cd3d8bbb72fac8600de41b12e73 |
| 2 | 7A_G | 7A_G.csv | 7 | A | G | 8e3447446c11b3cf33496de751a0e7b0 |
| 3 | 7A_K | 7A_K.csv | 7 | A | K | f94f0cf208f73ea5492f95cd8dc32ab2 |
| 4 | 7A_N | 7A_N.csv | 7 | A | N | 93596ed64b4c4495bbb95722f6a27415 |
| 5 | 7A_P | 7A_P.csv | 7 | A | P | 913a10ca78999596ad51db1321511d71 |
| 6 | 7B_E | 7B_E.csv | 7 | B | E | 51d8902b1dee08f1af3affe6510a87a5 |
Q: Why did I convert the trt variable to a factor?
For this analysis, we pick the data from team E
In [8]:
phdata<- phdata %>% filter(team=="E")
phdata
| sample | fname | trt | repl | team | md5 | |
|---|---|---|---|---|---|---|
| 1 | 7A_E | 7A_E.csv | 7 | A | E | 263e8cd3d8bbb72fac8600de41b12e73 |
| 2 | 7B_E | 7B_E.csv | 7 | B | E | 51d8902b1dee08f1af3affe6510a87a5 |
| 3 | 7C_E | 7C_E.csv | 7 | C | E | 49a549a26e58dd5c13bd931a794c0ec3 |
| 4 | 8A_E | 8A_E.csv | 8 | A | E | edaaefaf7ddeb1119f29cf3d5682d4ed |
| 5 | 8B_E | 8B_E.csv | 8 | B | E | f7d52ce9ffc0e61d7e371d072ebc0a43 |
| 6 | 8C_E | 8C_E.csv | 8 | C | E | 1e668998cb9947b80f3e813a7428bfb5 |
Now, we import the counts. Note that the first argument is the sample table while the second is the directory storing the count files. The last argument specifies the design. More on this later.
In [9]:
dds<-DESeqDataSetFromHTSeqCount(sampleTable=phdata,directory=datadir,design=~ trt)
Inspect object¶
Let’s has a look at the object we have created.
In [10]:
dds
class: DESeqDataSet
dim: 4444 6
metadata(1): version
assays(1): counts
rownames(4444): gene0 gene1 ... gene998 gene999
rowData names(0):
colnames(6): 7A_E 7B_E ... 8B_E 8C_E
colData names(4): trt repl team md5
Note that this object is of class DESeqDataSet.
In [11]:
class(dds)
The object dds has the following slots.
In [12]:
slotNames(dds)
- 'design'
- 'dispersionFunction'
- 'rowRanges'
- 'colData'
- 'assays'
- 'NAMES'
- 'elementMetadata'
- 'metadata'
To get a slot use @
In [13]:
dds@colData
DataFrame with 6 rows and 4 columns
trt repl team md5
<factor> <character> <character> <character>
7A_E 7 A E 263e8cd3d8bbb72fac8600de41b12e73
7B_E 7 B E 51d8902b1dee08f1af3affe6510a87a5
7C_E 7 C E 49a549a26e58dd5c13bd931a794c0ec3
8A_E 8 A E edaaefaf7ddeb1119f29cf3d5682d4ed
8B_E 8 B E f7d52ce9ffc0e61d7e371d072ebc0a43
8C_E 8 C E 1e668998cb9947b80f3e813a7428bfb5
Let’s look at the design
In [14]:
dds@design
~trt
The first thing you may want to do is to have a look at the raw counts you have imported. The DESeq2::counts function extracts a matrix of counts (with the genes along the rows and samples along the columns). Let us first verify the dimension of this matrix.
In [15]:
dim(counts(dds))
- 4444
- 6
Now print the raw counts for the first three genes (how can you verify this looking at the files from htseq-count)
In [16]:
head(counts(dds),3)
| 7A_E | 7B_E | 7C_E | 8A_E | 8B_E | 8C_E | |
|---|---|---|---|---|---|---|
| gene0 | 9 | 12 | 19 | 21 | 8 | 10 |
| gene1 | 108 | 119 | 155 | 193 | 164 | 196 |
| gene10 | 3 | 2 | 2 | 6 | 3 | 7 |
Slots of an S4 class¶
This gives the design of the study
In [17]:
dds@dispersionFunction
function ()
NULLThis slots return gene specific information (it will be populated later)
Estimate Size Factors and Dispersion Parameters¶
You recall that DESeq requires that we have estimates for sample specific size factors and gene specific dispersion factors. More specifically, recall that DESeq models the count \(K_{ij}\) (gene \(i\), sample \(j\)) as negative binomial with mean \(\mu_{ij}\) and dispersion parameter \(\alpha_i\). Here \(\mu_{ij}=s_j q_{ij}\) where \(\log_2(q_{ij}) = \beta_{0i} + \beta_{1i} z_j\). Here \(s_j\) is the sample \(j\) specific size factor.
Size Factors¶
We begin by estimating the size factors \(s_1,\ldots,s_n\):
In [18]:
dds <- estimateSizeFactors(dds)
Now, compare the dds object to that of before applying the estimateSizeFactors() function. What has changed? What remains unchanged?
In [19]:
dds
class: DESeqDataSet
dim: 4444 6
metadata(1): version
assays(1): counts
rownames(4444): gene0 gene1 ... gene998 gene999
rowData names(0):
colnames(6): 7A_E 7B_E ... 8B_E 8C_E
colData names(5): trt repl team md5 sizeFactor
Note that there is a sizeFactor added to colData. Let’s look at it more carefully
You can also get the size factors directly (why are there six size factors?)
In [20]:
sizeFactors(dds)
- 7A_E
- 0.814709325873651
- 7B_E
- 0.860242585687071
- 7C_E
- 0.982865560325398
- 8A_E
- 1.0938375107471
- 8B_E
- 1.15160914763126
- 8C_E
- 1.26477648704175
It is preferable to limit the number of decimal places. Next show the size factors rounded to 3 decimal places
In [21]:
round(sizeFactors(dds),3)
- 7A_E
- 0.815
- 7B_E
- 0.86
- 7C_E
- 0.983
- 8A_E
- 1.094
- 8B_E
- 1.152
- 8C_E
- 1.265
Now that the size factors have been estimated, we can get “normalized” counts
In [22]:
head(counts(dds),3)
head(counts(dds,normalize=TRUE),3)
| 7A_E | 7B_E | 7C_E | 8A_E | 8B_E | 8C_E | |
|---|---|---|---|---|---|---|
| gene0 | 9 | 12 | 19 | 21 | 8 | 10 |
| gene1 | 108 | 119 | 155 | 193 | 164 | 196 |
| gene10 | 3 | 2 | 2 | 6 | 3 | 7 |
| 7A_E | 7B_E | 7C_E | 8A_E | 8B_E | 8C_E | |
|---|---|---|---|---|---|---|
| gene0 | 11.046885 | 13.949554 | 19.331230 | 19.198464 | 6.946801 | 7.906535 |
| gene1 | 132.5626 | 138.3331 | 157.7021 | 176.4430 | 142.4094 | 154.9681 |
| gene10 | 3.682295 | 2.324926 | 2.034866 | 5.485275 | 2.605051 | 5.534575 |
Note that these are the counts divided by the size factors. Compare the first row of the last table (“normalized” counts for gene 1) to the hand calculation below.
In [23]:
counts(dds)[1,]/sizeFactors(dds)
- 7A_E
- 11.0468847160291
- 7B_E
- 13.9495535325256
- 7C_E
- 19.3312297906844
- 8A_E
- 19.1984639342426
- 8B_E
- 6.94680136611903
- 8C_E
- 7.90653534632788
Exercise: How do you get the raw counts for gene “GeneID:12930116”?
In [24]:
counts(dds)["gene10",]
- 7A_E
- 3
- 7B_E
- 2
- 7C_E
- 2
- 8A_E
- 6
- 8B_E
- 3
- 8C_E
- 7
Exercise: How do you get the normalized counts for gene gene10 for the first 10 sample?
In [25]:
counts(dds,normalize=TRUE)["gene10",]
- 7A_E
- 3.68229490534303
- 7B_E
- 2.32492558875426
- 7C_E
- 2.03486629375625
- 8A_E
- 5.48527540978361
- 8B_E
- 2.60505051229463
- 8C_E
- 5.53457474242952
Exercise: Get a summary (mean, median, quantiles etc ) of the size factors
In [26]:
summary(sizeFactors(dds))
Min. 1st Qu. Median Mean 3rd Qu. Max.
0.8147 0.8909 1.0380 1.0280 1.1370 1.2650
Before going to the next step, let’s look at the dispersionFunction slot
In [27]:
dds@dispersionFunction
function ()
NULLDispersion Parameters¶
Next, we get the dispersion factors \(\alpha_1,\ldots,\alpha_{m}\)
In [28]:
dds<-estimateDispersions(dds)
gene-wise dispersion estimates
mean-dispersion relationship
final dispersion estimates
Now inspect the dds object again and note that the rowRanges slot has extra information (“metadata column names(0):” before versus “column names(9): baseMean baseVar ... dispOutlier dispMAP”)
In [29]:
dds
class: DESeqDataSet
dim: 4444 6
metadata(1): version
assays(2): counts mu
rownames(4444): gene0 gene1 ... gene998 gene999
rowData names(9): baseMean baseVar ... dispOutlier dispMAP
colnames(6): 7A_E 7B_E ... 8B_E 8C_E
colData names(5): trt repl team md5 sizeFactor
Note that the dispersionfunction slot is now populated
In [30]:
dds@dispersionFunction
structure(function (q)
coefs[1] + coefs[2]/q, coefficients = structure(c(0.0296449332226886,
1.43330515554225), .Names = c("asymptDisp", "extraPois")), fitType = "parametric", varLogDispEsts = 1.18849739273446, dispPriorVar = 0.54356332588623)We can extract the gene specific dispersion factors using dispersions(). Note that there will be one number per gene. We look at the first four genes (rounded to 4 decimal places)
In [31]:
alphas<-dispersions(dds)
Verify that the number of dispersion factors equals the number of genes
In [32]:
length(alphas)
Print the dispersion factors for the first 5 genes rounded to four decimal points
In [33]:
round(alphas[1:5],4)
- 0.1363
- 0.0226
- 0.2759
- 0.0688
- 0.1377
Extract the metadata using mcols() for the first four genes
In [34]:
mcols(dds)[1:4,]
DataFrame with 4 rows and 9 columns
baseMean baseVar allZero dispGeneEst dispFit dispersion dispIter dispOutlier dispMAP
<numeric> <numeric> <logical> <numeric> <numeric> <numeric> <numeric> <logical> <numeric>
1 13.063245 29.15647 FALSE 0.127803253 0.13936539 0.13628195 6 FALSE 0.13628195
2 150.403062 256.01201 FALSE 0.003999823 0.03917469 0.02257188 8 FALSE 0.02257188
3 3.611165 2.47365 FALSE 0.000000010 0.42655433 0.27587228 8 FALSE 0.27587228
4 24.170850 35.51715 FALSE 0.014700541 0.08894385 0.06884450 8 FALSE 0.06884450
Exercise: Provide statistical summaries of the dispersion factors
In [35]:
summary(dispersions(dds))
Min. 1st Qu. Median Mean 3rd Qu. Max. NA's
0.01119 0.02864 0.05539 0.42150 0.17510 10.00000 130
Exercise: Summarize the dispersion factors using a box plot (may want to log transform)
In [36]:
boxplot(log(dispersions(dds)))

Differential Expression Analysis¶
We can now conduct a differential expression analysis using the DESeq() function. Keep in mind that to get to this step, we first estimated the size factors and then the dispersion parameters.
In [37]:
ddsDE<-DESeq(dds)
using pre-existing size factors
estimating dispersions
found already estimated dispersions, replacing these
gene-wise dispersion estimates
mean-dispersion relationship
final dispersion estimates
fitting model and testing
We can get the results for the differential expression analysis using results()
In [38]:
myres<-results(ddsDE)
Let’s look at the results for the first four genes
In [39]:
myres[1:4,]
log2 fold change (MAP): trt 8 vs 7
Wald test p-value: trt 8 vs 7
DataFrame with 4 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue padj
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric>
gene0 13.063245 -0.3615293 0.4895581 -0.7384809 0.4602223 0.6225190
gene1 150.403062 0.1396895 0.1984781 0.7038029 0.4815555 0.6402232
gene10 3.611165 0.5992853 0.6890620 0.8697117 0.3844580 0.5526493
gene100 24.170850 0.2231204 0.3708345 0.6016711 0.5473931 0.6965989
You can get the descriptions for the columns from the DE analysis
In [40]:
data.frame(desc=mcols(myres)$description)
| desc | |
|---|---|
| 1 | mean of normalized counts for all samples |
| 2 | log2 fold change (MAP): trt 8 vs 7 |
| 3 | standard error: trt 8 vs 7 |
| 4 | Wald statistic: trt 8 vs 7 |
| 5 | Wald test p-value: trt 8 vs 7 |
| 6 | BH adjusted p-values |
P-values¶
One can extract the unadjusted p-values as follows
In [41]:
pvalues<-myres$pvalue
length(pvalues)
pvalues[1:4]
- 0.460222295066648
- 0.481555502006739
- 0.384457970194728
- 0.547393065612409
The BH adjusted p-values can be extracted as
In [42]:
adjp<-myres$padj
length(adjp)
adjp[1:4]
- 0.622518959377389
- 0.640223160424753
- 0.552649254473344
- 0.696598867777664
Calculate BH adjusted P-values by “hand” using the p.adjust() function. Note that you will not replicate the results you get under the padj column (when looking at the first four rows)
In [43]:
pvalues<-myres$pvalue
BH<-p.adjust(pvalues,"BH")
data.frame(BH=BH[1:4],adjp=adjp[1:4])
| BH | adjp | |
|---|---|---|
| 1 | 0.670405 | 0.622519 |
| 2 | 0.6872980 | 0.6402232 |
| 3 | 0.6015538 | 0.5526493 |
| 4 | 0.7456737 | 0.6965989 |
The DESeq2::results function applies “independent” filtering. This enabled by default. Let’s disable and the reexamine the adjusted P-values
In [44]:
myres1<-results(ddsDE,independentFiltering = FALSE)
In [45]:
myres1
log2 fold change (MAP): trt 8 vs 7
Wald test p-value: trt 8 vs 7
DataFrame with 4444 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue padj
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric>
gene0 13.063245 -0.3615293 0.4895581 -0.7384809 0.46022230 0.67040501
gene1 150.403062 0.1396895 0.1984781 0.7038029 0.48155550 0.68729798
gene10 3.611165 0.5992853 0.6890620 0.8697117 0.38445797 0.60155379
gene100 24.170850 0.2231204 0.3708345 0.6016711 0.54739307 0.74567369
gene1000 7.675704 -1.1913393 0.5487654 -2.1709447 0.02993535 0.08914359
... ... ... ... ... ... ...
gene995 1.6197713 1.4706212 0.8162744 1.8016261 0.07160425 0.17719995
gene996 12.8488020 -0.2319250 0.4424331 -0.5242035 0.60013703 0.78035966
gene997 9.9461848 -1.0929507 0.4962577 -2.2023856 0.02763808 0.08386863
gene998 36.7337608 -0.2205173 0.3212546 -0.6864254 0.49244489 0.69827572
gene999 0.8127785 0.1074132 0.8147129 0.1318417 0.89510947 0.94515483
We can now replicate the results
In [46]:
pvalues1<-myres1$pvalue
BH1<-p.adjust(pvalues1[!is.na(pvalues)],"BH")
data.frame(BH=BH1[1:4],adjp=myres1$padj[1:4])
| BH | adjp | |
|---|---|---|
| 1 | 0.670405 | 0.670405 |
| 2 | 0.687298 | 0.687298 |
| 3 | 0.6015538 | 0.6015538 |
| 4 | 0.7456737 | 0.7456737 |
Subset and reorder the results¶
In [47]:
summary(myres,0.05)
out of 4314 with nonzero total read count
adjusted p-value < 0.05
LFC > 0 (up) : 615, 14%
LFC < 0 (down) : 685, 16%
outliers [1] : 8, 0.19%
low counts [2] : 501, 12%
(mean count < 2)
[1] see 'cooksCutoff' argument of ?results
[2] see 'independentFiltering' argument of ?results
You can sort the results by say the unadjusted P-values
In [48]:
myres[order(myres[["pvalue"]])[1:4],]
log2 fold change (MAP): trt 8 vs 7
Wald test p-value: trt 8 vs 7
DataFrame with 4 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue padj
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric>
gene1209 1110.3199 -3.347158 0.1492045 -22.43336 1.860541e-111 7.079359e-108
gene4405 436.6800 4.443710 0.2144262 20.72373 2.116499e-95 4.026639e-92
gene3312 488.4901 4.659130 0.2362760 19.71902 1.480824e-86 1.878178e-83
gene3317 322.3925 5.043525 0.2597286 19.41844 5.390243e-84 5.127469e-81
To get the list of genes with unadjusted P-values < 0.00001 and absolute log2 FC of more than 4
In [49]:
subset(myres,pvalue<0.00001&abs(log2FoldChange)>4)
log2 fold change (MAP): trt 8 vs 7
Wald test p-value: trt 8 vs 7
DataFrame with 24 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue padj
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric>
gene1300 175.91020 4.761123 0.4248758 11.20592 3.813880e-29 3.023294e-27
gene2034 128.44386 4.061622 0.3738799 10.86344 1.721393e-27 1.235830e-25
gene2127 82.92247 4.353625 0.3826230 11.37837 5.359438e-30 4.338864e-28
gene2128 120.48719 4.474566 0.3303883 13.54335 8.673550e-42 1.434907e-39
gene2179 436.50102 5.055035 0.2805605 18.01763 1.416826e-72 1.078204e-69
... ... ... ... ... ... ...
gene4405 436.68000 4.443710 0.2144262 20.723727 2.116499e-95 4.026639e-92
gene585 27.68248 5.127728 0.6402093 8.009455 1.152179e-15 3.535517e-14
gene594 121.06565 5.862144 0.4591564 12.767207 2.499646e-37 3.396840e-35
gene595 106.31889 4.798168 0.3853057 12.452885 1.348798e-35 1.466336e-33
gene596 69.53289 4.007674 0.4010769 9.992284 1.647420e-23 9.084685e-22
To get the list of genes with unadjusted P-values < 0.00001 and upregulated genes with log2 FC of more than 4
In [50]:
subset(myres,pvalue<0.00001&log2FoldChange>4)
log2 fold change (MAP): trt 8 vs 7
Wald test p-value: trt 8 vs 7
DataFrame with 24 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue padj
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric>
gene1300 175.91020 4.761123 0.4248758 11.20592 3.813880e-29 3.023294e-27
gene2034 128.44386 4.061622 0.3738799 10.86344 1.721393e-27 1.235830e-25
gene2127 82.92247 4.353625 0.3826230 11.37837 5.359438e-30 4.338864e-28
gene2128 120.48719 4.474566 0.3303883 13.54335 8.673550e-42 1.434907e-39
gene2179 436.50102 5.055035 0.2805605 18.01763 1.416826e-72 1.078204e-69
... ... ... ... ... ... ...
gene4405 436.68000 4.443710 0.2144262 20.723727 2.116499e-95 4.026639e-92
gene585 27.68248 5.127728 0.6402093 8.009455 1.152179e-15 3.535517e-14
gene594 121.06565 5.862144 0.4591564 12.767207 2.499646e-37 3.396840e-35
gene595 106.31889 4.798168 0.3853057 12.452885 1.348798e-35 1.466336e-33
gene596 69.53289 4.007674 0.4010769 9.992284 1.647420e-23 9.084685e-22
The P-values for the four top genes are beyond machine precision. You can use the format.pval() function to properly format the P-values. PLEASE promote ending the practice of publishing P-values below machine precision. (that would be akin to stating the weight of an object that weighs less than one pound with scale that whose minimum weight spec is 1lbs).
In [51]:
myres$pval=format.pval(myres$pvalue)
myres[order(myres[["pvalue"]])[1:4],]
log2 fold change (MAP): trt 8 vs 7
Wald test p-value: trt 8 vs 7
DataFrame with 4 rows and 7 columns
baseMean log2FoldChange lfcSE stat pvalue padj pval
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric> <character>
gene1209 1110.3199 -3.347158 0.1492045 -22.43336 1.860541e-111 7.079359e-108 < 2.22e-16
gene4405 436.6800 4.443710 0.2144262 20.72373 2.116499e-95 4.026639e-92 < 2.22e-16
gene3312 488.4901 4.659130 0.2362760 19.71902 1.480824e-86 1.878178e-83 < 2.22e-16
gene3317 322.3925 5.043525 0.2597286 19.41844 5.390243e-84 5.127469e-81 < 2.22e-16
Let’s look at a volcano plot
In [52]:
plot(myres$log2FoldChange,-log10(myres$padj),pch=19,cex=0.3,xlab="Log2 FC",ylab="-log10(BH Adjusted P-value)")
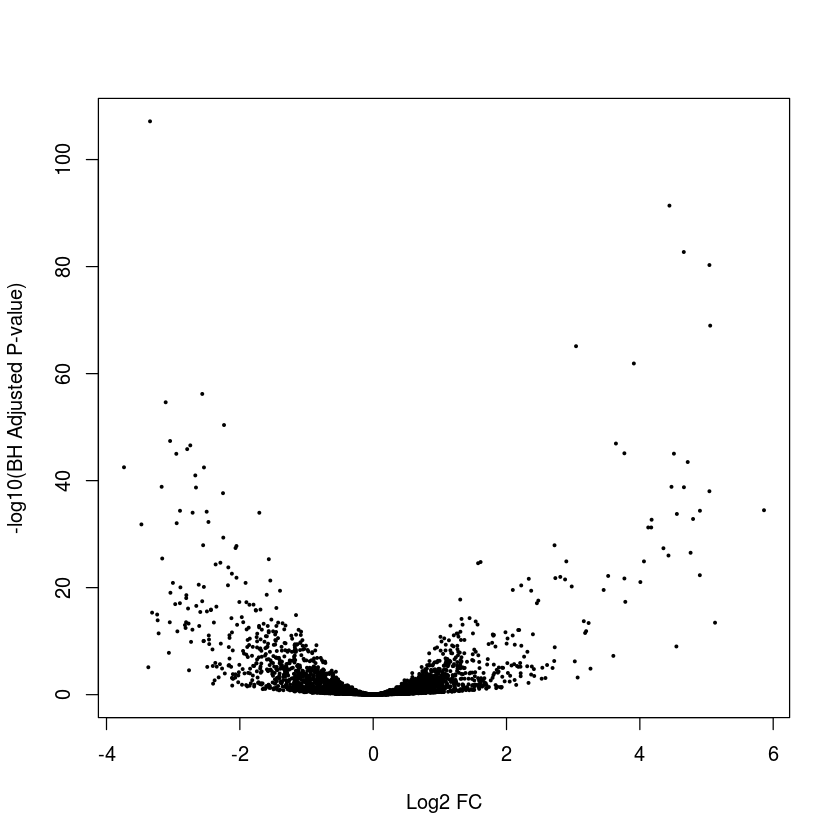
Extract results for genes GeneID:12932226 and GeneID:12930116
In [53]:
myres[c("gene3317","gene10"),]
log2 fold change (MAP): trt 8 vs 7
Wald test p-value: trt 8 vs 7
DataFrame with 2 rows and 7 columns
baseMean log2FoldChange lfcSE stat pvalue padj pval
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric> <character>
gene3317 322.392539 5.0435248 0.2597286 19.4184401 5.390243e-84 5.127469e-81 < 2.22e-16
gene10 3.611165 0.5992853 0.6890620 0.8697117 3.844580e-01 5.526493e-01 0.38445797
Exercise: Annotate the hits with adjusted P-values < 0.05 and absolute log2 FC greater than 2 in red
In [54]:
plot(myres$log2FoldChange,-log10(myres$padj),pch=19,cex=0.3,xlab="Log2 FC",ylab="-log10(BH Adjusted P-value)",col=ifelse(myres$padj<0.05&abs(myres$log2FoldChange)>2,"red","black"))

Converting/Normalizing Counts to “Expressions”¶
Normalized Counts¶
We have already shown how to “normalize” the counts using the estimated size factors
In [55]:
head(counts(dds,normalize=TRUE),3)
| 7A_E | 7B_E | 7C_E | 8A_E | 8B_E | 8C_E | |
|---|---|---|---|---|---|---|
| gene0 | 11.046885 | 13.949554 | 19.331230 | 19.198464 | 6.946801 | 7.906535 |
| gene1 | 132.5626 | 138.3331 | 157.7021 | 176.4430 | 142.4094 | 154.9681 |
| gene10 | 3.682295 | 2.324926 | 2.034866 | 5.485275 | 2.605051 | 5.534575 |
Plot the counts stratified by treatment for the 2nd gene
In [56]:
plotCounts(dds, 2,intgroup="trt")
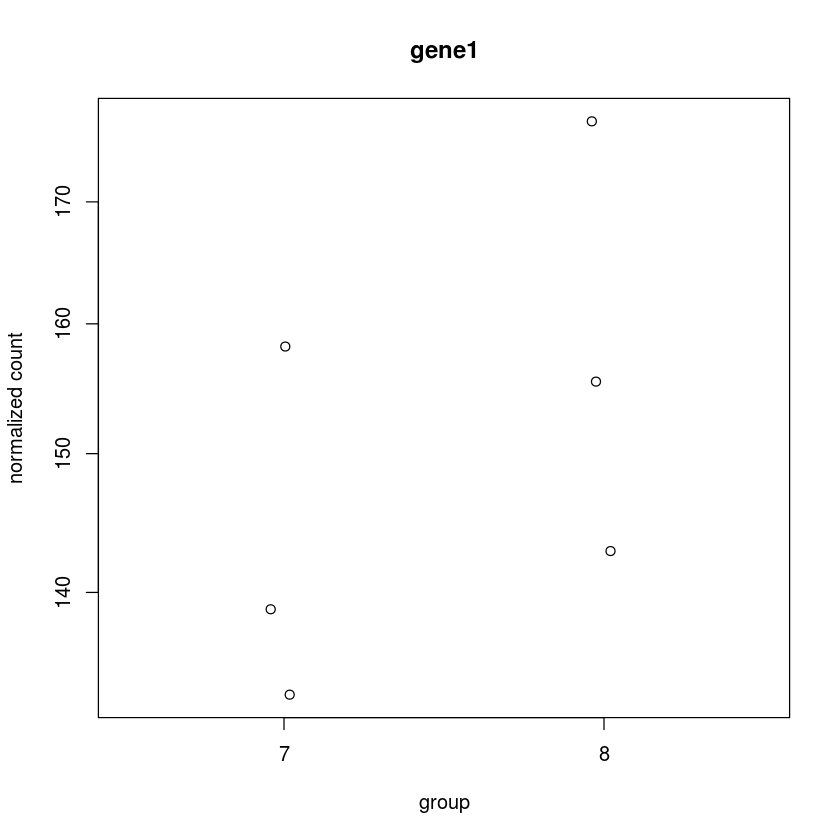
Or alternatively (better)
In [57]:
plotCounts(dds, "gene10",intgroup="trt")
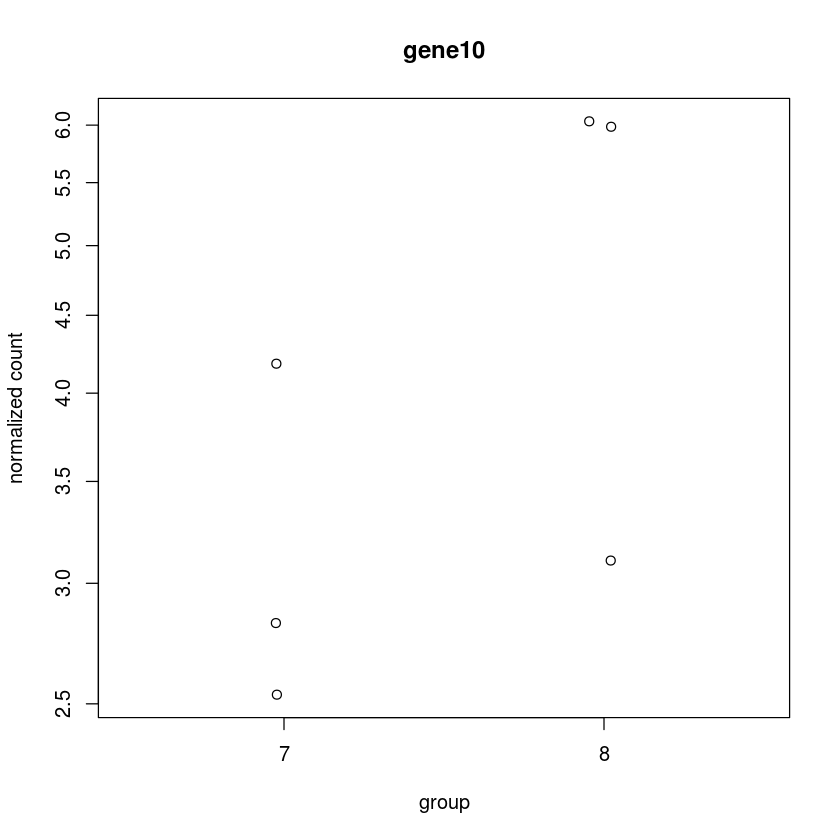
Now get this plot for the top hit
In [58]:
plotCounts(dds, "gene1209",intgroup="trt")
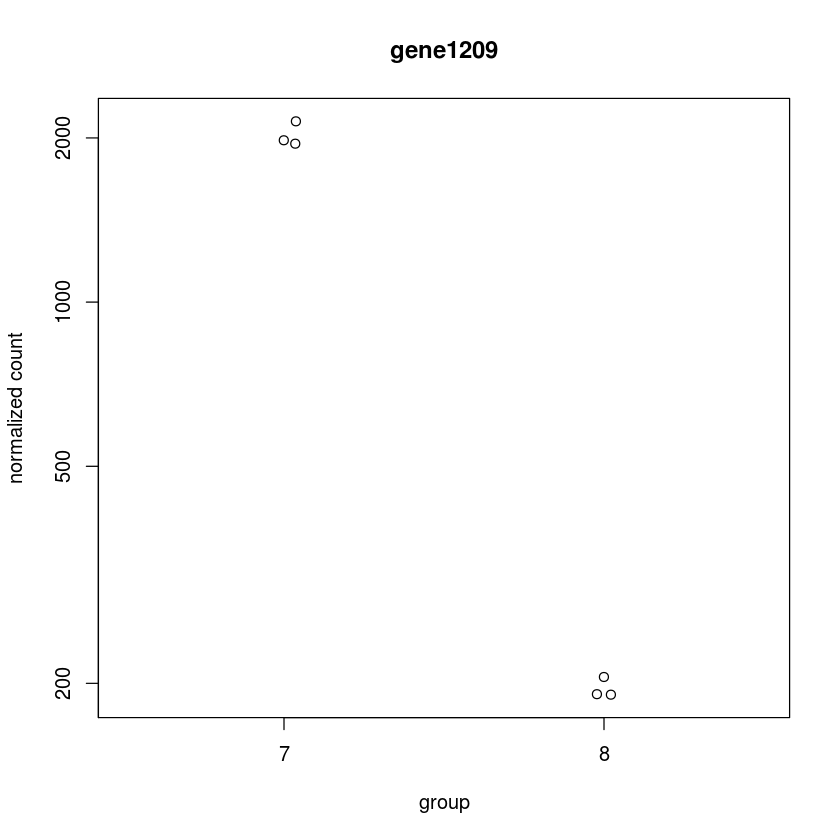
FPM¶
Another approach is to FPM: fragments per million mapped fragments
In [59]:
head(fpm(dds),3)
| 7A_E | 7B_E | 7C_E | 8A_E | 8B_E | 8C_E | |
|---|---|---|---|---|---|---|
| gene0 | 7.563666 | 9.551087 | 13.235854 | 13.144951 | 4.756389 | 5.413507 |
| gene1 | 90.76399 | 94.71495 | 107.97671 | 120.80836 | 97.50598 | 106.10474 |
| gene10 | 2.521222 | 1.591848 | 1.393248 | 3.755700 | 1.783646 | 3.789455 |
Let’s calculate the FPM manually. For gene \(i\) sample \(j\), the FPM is defined as \(\frac{K_{ij}}{D_j}\times 10^{6}\) where \(D_j=\sum_{i=1} K_{ij}\) is the read depth for sample \(j\). First get the read depth for each sample
In [60]:
D<-colSums(counts(dds))
D
- 7A_E
- 1224153
- 7B_E
- 1294576
- 7C_E
- 1493765
- 8A_E
- 1492923
- 8B_E
- 1581586
- 8C_E
- 1736478
By default, the fpm() function uses a robust approach. We will disable this right now as to replicate the standard FPM. Let’s look at gene 1
In [61]:
fpm1<-fpm(dds,robust=FALSE)[1,]
fpm1
- 7A_E
- 7.35202217369888
- 7B_E
- 9.26944420412552
- 7C_E
- 12.7195375443929
- 8A_E
- 14.0663651105918
- 8B_E
- 5.05821371711687
- 8C_E
- 5.75878300790451
Now get the raw counts for gene 1
In [62]:
cnt1<-counts(dds)[1,]
cnt1
- 7A_E
- 9
- 7B_E
- 12
- 7C_E
- 19
- 8A_E
- 21
- 8B_E
- 8
- 8C_E
- 10
Now calculate the FPM for gene 1
In [63]:
myfpm1<-cnt1/D*1e6
myfpm1
- 7A_E
- 7.35202217369888
- 7B_E
- 9.26944420412552
- 7C_E
- 12.7195375443929
- 8A_E
- 14.0663651105918
- 8B_E
- 5.05821371711687
- 8C_E
- 5.75878300790451
This is how you check if two numeric columns are “equal”? One approach is to calculate the maximum absoute difference
In [64]:
max(abs(fpm1-myfpm1))
The above approach is also helpful in establishing if the difference is “small”. Another approach to test for equality to use the all.equal() function
In [65]:
all.equal(fpm1,myfpm1)
It is generally a bad idea to compare numeric vectors using == (e.g., fpm1==myfpm1)
FPKM¶
To calculate the FPKM (fragments per kilobase per million mapped fragments) we need to add annotation to assign the feature lengths. More specifically, for gene \(i\) sample \(j\), the FPKM is defined as \(\frac{K_{ij}}{\ell_i D_j}\times 10^3 \times 10^{6}\) where \(\ell_i\) is the “length” of gene \(i\) (fragments for each \(10^3\) bases in the gene for every \(\frac{D_j}{10^6}\) fragments. More on this later.
Regularized log transformation¶
The regularized log transform can be obtained using the rlog() function. Note that an important argument for this function is blind (TRUE by default). The default “blinds” the normalization to the design. This is very important so as to not bias the analyses (e.g. class discovery)
In [66]:
rld<-rlog(dds,blind=TRUE)
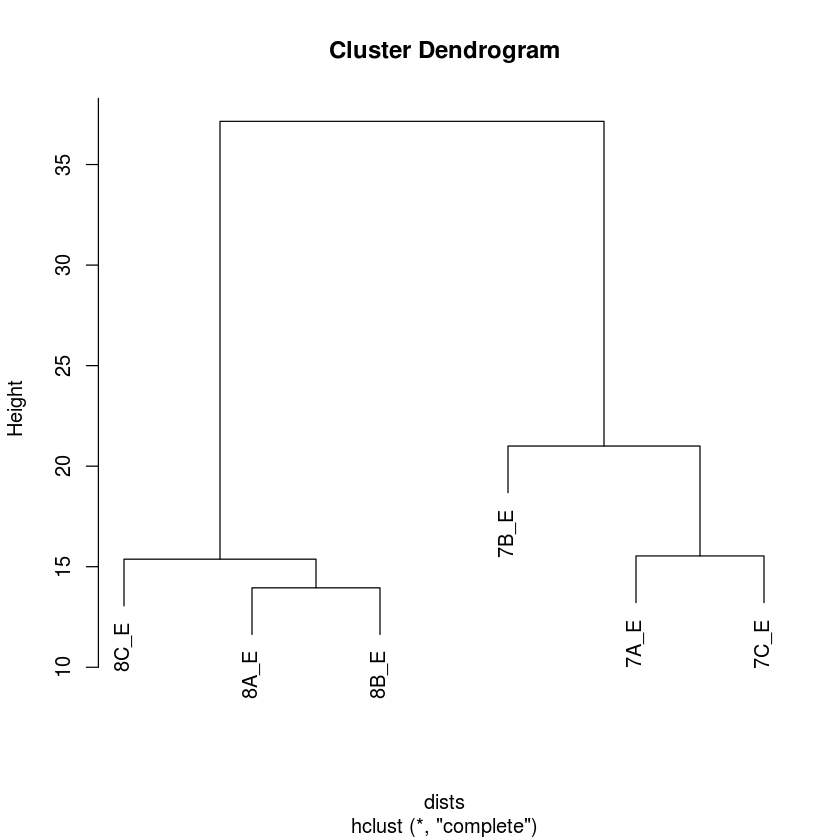
Hierarchical clustering using rlog transformation
In [67]:
dists<-dist(t(assay(rld)))
plot(hclust(dists))
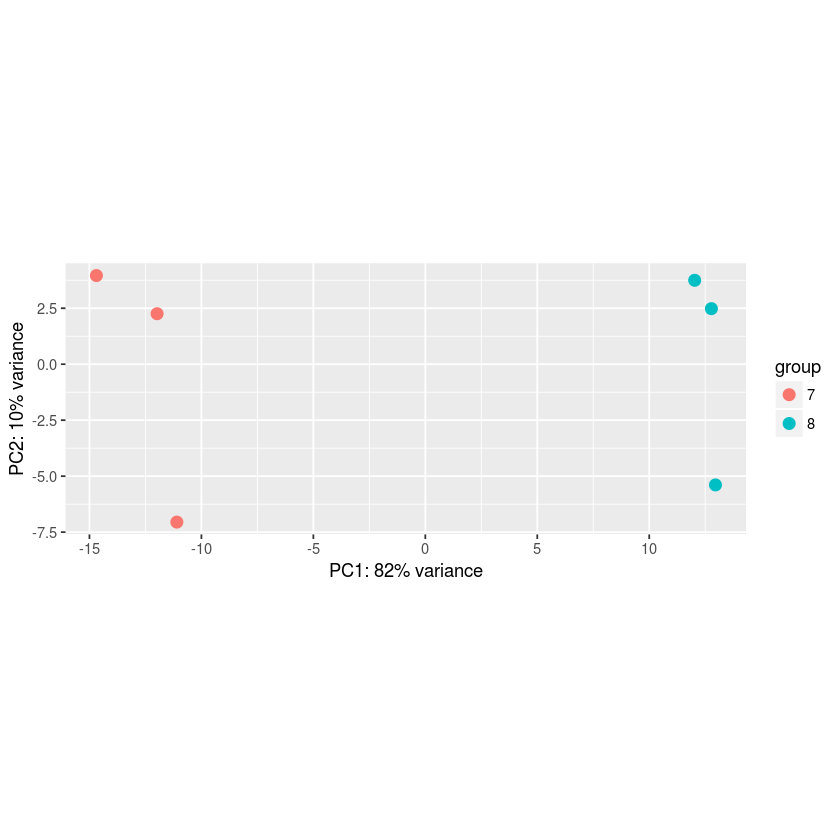
PC Analysis using the rlog transformation
In [68]:
plotPCA(rld,intgroup="trt")
Variance Stabilizing Transformation (vst) and mean-variance modelling at the observational level (voom)¶
Two other normalization approaches for RNA-Seq count data are provided by the functions DESeq2::vst and limma::voom (note that for the latter one needs the limma package).
From ? DESeq2::vst “This function calculates a variance stabilizing transformation (VST) from the fitted dispersion-mean relation(s) and then transforms the count data (normalized by division by the size factors or normalization factors), yielding a matrix of values which are now approximately homoskedastic (having constant variance along the range of mean values). The transformation also normalizes with respect to library size.”
Compared to DESeq2::rlog “The ‘rlog’ is less sensitive to size factors, which can be an issue when size factors vary widely. These transformations are useful when checking for outliers or as input for machine learning techniques such as clustering or linear discriminant analysis.”
From ? limma::voom “Transform count data to log2-counts per million (logCPM), estimate the mean-variance relationship and use this to compute appropriate observation-level weights. The data are then ready for linear modelling.”
Get VST transformation
In [69]:
VST<-vst(dds)
class(VST)
Get the VST matrix
In [70]:
VSTmat<-assay(VST)
dim(VSTmat)
VSTmat[1:10,]
- 4444
- 6
| 7A_E | 7B_E | 7C_E | 8A_E | 8B_E | 8C_E | |
|---|---|---|---|---|---|---|
| gene0 | 3.923730 | 4.180475 | 4.559309 | 4.551088 | 3.452826 | 3.578618 |
| gene1 | 7.097850 | 7.157394 | 7.340988 | 7.498825 | 7.198022 | 7.316447 |
| gene10 | 2.901962 | 2.572007 | 2.486853 | 3.234850 | 2.648356 | 3.242828 |
| gene100 | 5.077572 | 4.083261 | 4.843068 | 4.809872 | 4.964222 | 4.998826 |
| gene1000 | 4.143823 | 3.978753 | 3.607448 | 3.077093 | 2.856875 | 3.108463 |
| gene1001 | 2.203473 | 2.859947 | 2.760425 | 3.077093 | 2.856875 | 3.108463 |
| gene1002 | 5.446930 | 5.335202 | 4.736260 | 4.945204 | 4.175947 | 4.678644 |
| gene1003 | 6.680922 | 6.914822 | 6.352627 | 6.582342 | 6.552185 | 6.574850 |
| gene1004 | 2.203473 | 1.155868 | 2.113095 | 2.064865 | 1.155868 | 2.003037 |
| gene1005 | 10.071462 | 10.519630 | 9.903560 | 8.758925 | 9.039811 | 8.859864 |
Get voom transformation (note that according to ? limma::voom, the function is expecting raw counts
In [71]:
VOOM<-limma::voom(counts(dds))
Get the VOOM matrix
In [72]:
VOOMmat<-VOOM$E
dim(VOOMmat)
VOOMmat[1:10,]
- 4444
- 6
| 7A_E | 7B_E | 7C_E | 8A_E | 8B_E | 8C_E | |
|---|---|---|---|---|---|---|
| gene0 | 2.956142 | 3.271375 | 3.706448 | 3.848124 | 2.426090 | 2.596152 |
| gene1 | 6.469766 | 6.528386 | 6.701817 | 7.018049 | 6.700571 | 6.822221 |
| gene10 | 1.5155699 | 0.9494473 | 0.7429739 | 2.1222990 | 1.1459820 | 2.1107256 |
| gene100 | 4.322925 | 3.151081 | 4.035756 | 4.149780 | 4.315907 | 4.353582 |
| gene1000 | 3.231777 | 3.019837 | 2.508509 | 1.881291 | 1.508552 | 1.904275 |
| gene1001 | 0.2931774 | 1.4348741 | 1.2284008 | 1.8812909 | 1.5085521 | 1.9042748 |
| gene1002 | 4.730583 | 4.604799 | 3.912899 | 4.304502 | 3.383021 | 3.985195 |
| gene1003 | 6.039132 | 6.278571 | 5.678434 | 6.072911 | 6.032114 | 6.055584 |
| gene1004 | 0.293177439 | -1.372480778 | 0.006008335 | 0.006821776 | -1.661372918 | -0.211202463 |
| gene1005 | 9.478879 | 9.926155 | 9.293721 | 8.296841 | 8.571048 | 8.389949 |
Get Session Information¶
In [73]:
sessionInfo()
R version 3.3.1 (2016-06-21)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Debian GNU/Linux 8 (jessie)
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C LC_TIME=en_US.UTF-8
[4] LC_COLLATE=en_US.UTF-8 LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C LC_ADDRESS=C
[10] LC_TELEPHONE=C LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] tools parallel stats4 stats graphics grDevices utils datasets methods
[10] base
other attached packages:
[1] dplyr_0.5.0 qvalue_2.6.0 limma_3.30.13
[4] DESeq2_1.14.1 SummarizedExperiment_1.4.0 Biobase_2.34.0
[7] GenomicRanges_1.26.4 GenomeInfoDb_1.10.3 IRanges_2.8.2
[10] S4Vectors_0.12.2 BiocGenerics_0.20.0
loaded via a namespace (and not attached):
[1] locfit_1.5-9.1 Rcpp_0.12.8 lattice_0.20-34 assertthat_0.1
[5] digest_0.6.10 IRdisplay_0.4.3 R6_2.2.0 plyr_1.8.4
[9] repr_0.7 backports_1.1.0 acepack_1.4.1 RSQLite_1.0.0
[13] evaluate_0.10 ggplot2_2.2.1 zlibbioc_1.20.0 lazyeval_0.2.0
[17] uuid_0.1-2 data.table_1.10.4 annotate_1.52.1 rpart_4.1-10
[21] Matrix_1.2-7.1 checkmate_1.8.3 labeling_0.3 splines_3.3.1
[25] BiocParallel_1.8.2 geneplotter_1.52.0 stringr_1.0.0 foreign_0.8-67
[29] htmlwidgets_0.9 RCurl_1.95-4.8 munsell_0.4.3 base64enc_0.1-3
[33] htmltools_0.3.5 nnet_7.3-12 tibble_1.2 gridExtra_2.2.1
[37] htmlTable_1.9 Hmisc_4.0-3 XML_3.98-1.9 crayon_1.3.1
[41] bitops_1.0-6 grid_3.3.1 jsonlite_1.1 xtable_1.8-2
[45] gtable_0.2.0 DBI_0.5-1 magrittr_1.5 scales_0.4.1
[49] stringi_1.1.2 reshape2_1.4.2 XVector_0.14.1 genefilter_1.56.0
[53] latticeExtra_0.6-28 IRkernel_0.7 Formula_1.2-2 RColorBrewer_1.1-2
[57] survival_2.41-3 AnnotationDbi_1.36.2 colorspace_1.3-1 cluster_2.0.5
[61] memoise_1.0.0 pbdZMQ_0.2-3 knitr_1.15.1
In [ ]: