3. 5 Photosynthesis
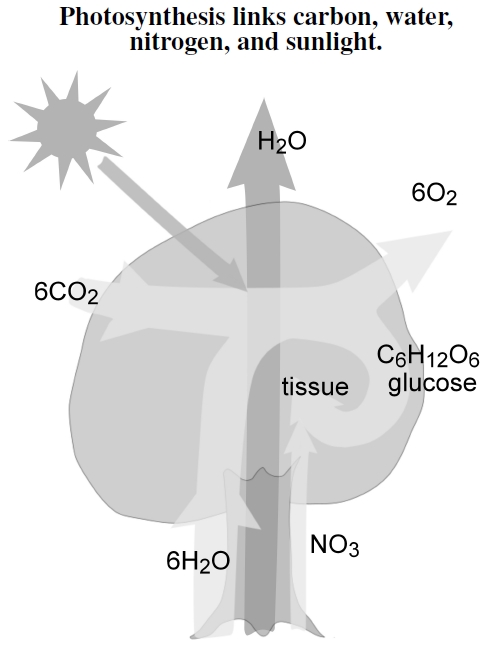
Figure 3.5: Plants take in carbon dioxide (CO2) from the atmosphere and water (H2O) from the soil, and use solar energy to produce a variety of energy storage molecules, including the sugar glucose (C6H12O6). Behind much of these photosynthetic processes, plants transpire lots of water to transport soil nutrients like nitrogen (NO3−) to build proteins.
Our lives completely depend on photosynthesis. Figure 3.5 shows its essential features, linking many features I discuss later. Photosynthesis pulls carbon dioxide from the atmosphere and water from the ground, transforming sunlight into sugar to store energy. Plants metabolize the sugar — just like their herbivorous and frugivorous consumers — and recover the energy, using it to make all sorts of biological material, including the volatile organic compounds (VOCs) emitted along with oxygen, O2. Besides solar energy and carbon dioxide (CO2), tissue-making depends on many other chemical compounds and elements. Pulled up along with transpired water from the soil, most plants need, for example, nitrate, NO3− , to build nitrogen-containing amino acids, the building blocks of proteins, and nucleotides, the building blocks of RNA and DNA.[25] By dry weight, plants comprise about 44% carbon, 44% oxygen, 6% hydrogen, 1-4% nitrogen, 0.5-6% potassium, 0.2-3.5% calcium, 0.1-0.8% phosphorous, and then a slew of minor but often essential elements.[26] In the end, we eat and burn these sugars and tissues, making our lives completely dependent on past and present solar power, save for small nuclear energy contributions.
Plants recycle lots of carbon in a very short amount of time.[27] The atmosphere contains around 750 × 1015 grams — 750 trillion kg — of carbon, but in a single year plants fix roughly 120 trillion kg into their tissues and oceans absorb 90 trillion kg. Plants, animals, fungi and bacteria consume living and dead tissues of plants, and, for the most part, respire an equal amount of carbon back into the atmosphere, offsetting the carbon fixed by vegetation. That’s a huge amount of recycling: A carbon atom, on average spends only about four years in the atmosphere (750 trillion kg)/(210 trillion kg/year) before, with a good chance, being pulled back into some plant’s stomata and fixed into its living tissues. On top of these amounts, humans put 6 × 1015 grams of fossil-fuel carbon into the atmosphere each year, a mere 6 trillion kg, or just 5% of the natural fixation by plants.
The biosphere buries just a small fraction of its vegetation. Multiplied over hundreds of millions of years, this buried plant material amounts to significant deposits of hydrocarbons. Over the last century or two (see Figure 3.1), we used fuels from these buried and concentrated fossil plants, releasing carbon long ago pulled from the atmosphere and sequestered out of the biosphere. It’s ironic that we’re trying to both use the solar energy stored in those fossil hydrocarbons and subsequently sequester the released carbon dioxide. If we just stopped using the solar power contained in these fossil fuels in favor of direct solar power, we could stop worrying about carbon sequestration.
—————————–
[25]All amino acids contain nitrogen, and in long molecular chains, called polypeptides, make up proteins that fold up into chemically active components of biological processes. Parts of RNA (ribonucleic acid) and DNA (deoxyribonucleic acid) molecules are composed of nucleotide building blocks, each built out of a sugar molecule, a phosphorous-containing group of atoms, and a nitrogen-containing group of atoms.
[26]The Biology of Plants by Raven, Evert, and Eichhorn, discusses the elemental composition of plants. These few atoms comprise about 98% or more of nearly all organisms’ weight.
[27]Carbon pools and fluxes are very nicely described in Chapter 11 of the biogeochemistry book by Schlesinger (1997).