4. 1 Human VOCs
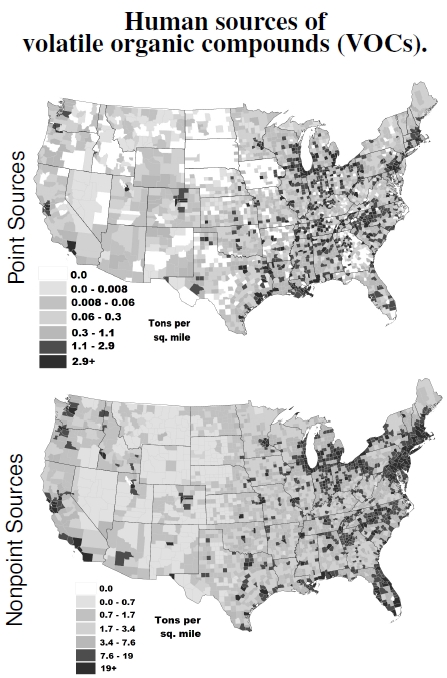
Figure 4.1: Human sources of volatile organic compounds (VOCs) distributed across the contiguous United States in 2001 (images from U.S. Environmental Protection Agency). Point sources (top image) include power plants and factories with high emissions, while nonpoint sources (bottom image) cover vehicles, dry cleaners, and the many small emission sources too numerous to inventory individually. Note the different scales on the two sources. (1 ton/mi2/yr = 40μg/m2/hr.)
Figure 4.1 shows human-caused emissions of volatile organic compounds (VOCs) across the United States.[1] Point sources represent power plants and factories, things that stay fixed on the ground, generating emissions from a single location, and, perhaps, above some emission level.[2] Point source emissions take place close to cities in part because people want electricity and long transmission lines waste electricity. And they place not just anywhere close. Evidence exists that these point sources are more often sited near poor and minority communities.[3]
Nonpoint sources clearly are located where people live and, more importantly, drive. These sources move around on the ground, not just cars and trucks, but also trains, planes, and tractors — off-road vehicles that add up to a lot of emissions. Take a look back at the distribution of people across the 48 states in Figure 1.1 and compare with these emissions figures. A pretty strong correlation exists because the EPA uses population densities to come up with some estimates for nonpoint sources, assuming there’s an average emissions per person. One can’t argue too much with that approach, given constrained budgets for air sampling and the fact that 300 million Americans use spray paint and hair care products (and the like), all included in nonpoint sources.
What compounds are we talking about here? There’s a slew of chemicals, but the principal components of both urban air and car exhaust include ethane (C2H6), ethylene (C2H4), propylene (C3H6), propane (C3H8), butane (C4H10), iso-pentane (C5H12), benzene (C6H6), and toluene (C7H8).[4] All of these chemicals can be substituted for the methane (CH4) in Figure 4.7, factoring into the production of ground-level ozone.
Another important ingredient of urban air comes from burning fossil fuels: It emits a pair of nitrogen oxide compounds, NO+NO2,[5] nitric oxide and nitrogen dioxide, respectively, and these reactive forms of nitrogen, together called NOx, cause problems in our urban air.[6] Though reactive nitrogen right near the source of its emission depresses ozone concentrations (see Figure 4.14) through the reactions outlined in Figure 4.7,[7] NOx remains after removing the ozone, and later reactions result in downwind ozone increases during daylight hours (see Figure 4.8).[8]
We’ll examine a few of these complicated emission issues and their consequences when we look at ozone production later in this chapter.
—————————————
[1]The human point and nonpoint VOC source images from the U.S. EPA were produced from a utility at www.epa.gov.
[2]One document, for example, on Louisiana’s Emission Inventory defines point sources as “stationary commercial or industrial operations that emit 100 tons or more per year of VOC or NOx.”
[3]Maantay (2007) examines the connection between point source emissions and minority populations in New York City.
[4]De Gouw et al. (2005) provide an inventory of the volatile organic compounds present in the emissions of fossil fuels in the New England area of North America.
[5]I briefly discussed agricultural nitrogen in Figure 1.6. Nitrogen causes problems, but don’t fear all of it. In the form of N2, it makes up some 78% of our atmosphere. That form of molecular nitrogen has a strong triple bond joining the two atoms together, which few biological processes can tear apart. As a result, that triple bond makes the N2 molecule nearly irrelevant to issues discussed here.
[6]Olszyna et al. (1997) provide a detailed discussion of nitrogen emissions and ozone production in the southeastern United States.
[7]Evolution of these plumes has been studied mostly during the day when chemical reactions, spurred on by light and atmospheric mixing by winds, take place. See Figure 4.7.
[8]Gillani and Pleim (1996) nicely cover anthropogenic VOC emissions.