4. 7 VOCs & Ozone
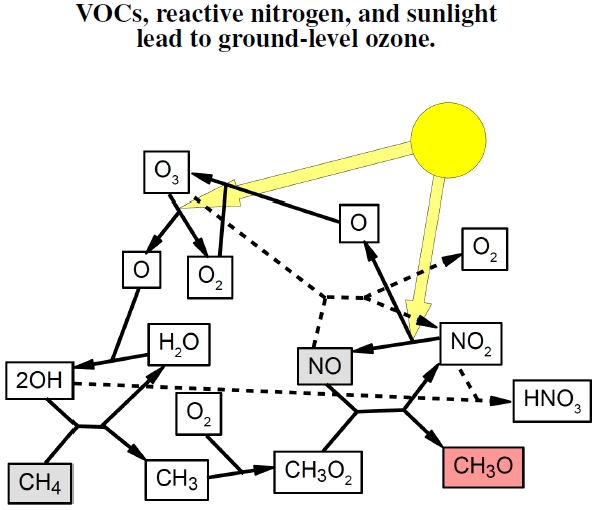
Figure 4.7: Summary of the reactions linking volatile organic compounds (VOCs), nitrogen emissions, and ozone. Light gray boxes represent VOCs, like methane, CH4, and nitric oxide, NO (a result of burning fossil fuels). High ozone levels can arise from both types of emissions on warm, sunny days. Light splits nitrogen dioxide, NO2, freeing an oxygen atom to react with an oxygen molecule, O2, producing ozone, O3. At the same time, light, as well as interactions with nitric oxide (dashed lines), degrades ozone. Many factors, including the relative concentrations of VOCs and reactive nitrogen involved in the competition for OH, determine ozone levels.
Let’s put these emissions into the unifying context of atmospheric chemistry. Our atmosphere is one big beaker of chemicals, lit up and heated by the Sun, stirred by the weather, with humans adding substantial novel and bizarre pollutants, as well as breathing it in and out of their lungs. This plot caricatures ground-level ozone (O3) production resulting from this big atmospheric stew.[39]
Ozone can be good or bad, depending on where it floats. Ozone in the stratosphere — way up above 10 km — protects the biosphere from damage by high-energy, short-wavelength sunlight. Ozone in the troposphere — way down here near the ground — does direct damage to living organisms (see Figure 4.13).
In Figure 4.7, I represent by the light gray squares both emissions by people burning fossil fuels and naturally occurring VOCs, and final products by the dark gray square. My example uses methane, CH4, and other chemical names listed include O: oxygen; O2: oxygen, or diatomic oxygen; H2O: water; OH: hydroxyl molecule; NO: nitric oxide; NO2: nitrogen dioxide; HNO3: nitric acid; and CH3O: the methoxy radical. Along with the ground-level ozone, these final products cause a variety of discomforts.[40] I’ll focus quite heavily on asthma and heart problems in the next chapter.
Of course, my diagram greatly simplifies the picture, yet it provides the essentials for the relevant depth of understanding needed to help interpret other results presented in this chapter.[41] Where to start? Oxygen, O2, gets split by high-energy photons, light with a short wavelength less than 240 nm,[42] into two oxygen atoms; each one can then react with another O2 molecule to form ozone, O3. Ozone can cleave back into O2 and an O with light around 240-320 nm being the cleaver.[43] In the early morning, most of the nitrogen exists as NO2, with ozone removed during the nighttime by the reactions with NO, represented by the dashed lines. Once sunlight starts acting on the system, however, photochemical reactions make OH and NO available, which subsequently promotes ozone production.
Although I use methane as the input VOC, any old VOC will do, including the biogenically produced isoprene, C5H8. Indeed, the hydroxyl ion, OH, reacts thousands of times faster with isoprene molecules than with either CO or methane, meaning that high levels of isoprene enhance ozone formation compared with these other molecules. In this way, biogenic VOCs can have large impacts on ozone formation even when NO from fossil fuel emissions are abundant.
—————————–
[39]Many atmospheric chemistry books exist; I found the one by Seinfeld and Pandis (2006) helpful.
[40]Though I list CH3O, the methoxy radical, as the final product of this example using the VOC methane, formaldehyde, CH2O, actually constitutes an intermediate result, which lasts about four hours in the atmosphere, producing carbon monoxide, CO, which lasts several months, ultimately ending up as carbon dioxide, CO2. I skipped these steps because many other chemicals get involved, and the figure gets really messy.
[41]Baldocchi et al. (1995) provide another full description of ozone formation in the presence of VOCs.
[42]A nanometer (nm) equals 10−9 meters. Visible light has wavelengths ranging from 400 to 700 nm, whereas, for example, radio waves (another form of light) have wavelengths of a meter to thousands of meters and very low energy per photon.
[43]The common mnemonic to remember and distinguish frequencies and wavelengths of the visible light spectrum is ROY G. BIV, for red, orange, yellow, green, blue, indigo, and violet. Further, the letter “V” looks like the greek letter ν, the symbol for the frequency of light. Because the V is at the mnemonic’s end, violet has the highest frequency. Ultraviolet has an even higher frequency, and infrared — below red — has a low frequency. The energy of a photon of light is proportional to its frequency, and it’s the ultraviolet light with high frequency that causes damage. The speed of light, the product of the frequency and the wavelength, is the same for the entire spectrum. Thus, high-frequency ultraviolet light has a short wavelength, and low-frequency infrared light has a long wavelength.
Here’s an erratum.
Above I wrote: “Oxygen, O2, gets split by high energy photons, light with a short wavelength less than 240 nm, into two oxygen atoms, each one can then react with another O2 molecule to form ozone, O3.”
While correct for the stratosphere, it’s not an important source of O atoms for ozone formation in the troposphere because the atmosphere blocks the relevant portion of the solar spectrum (wavelengths less than 240 nm). The light certainly isn’t present in the diagram shown here for tropospheric ozone formation, so I somehow messed up this text.
The least disruptive edit replaces that sentence with: “Oxygen, O2, reacts with an oxygen atom to form ozone, O3.”
Trying to figure this mistake out, I found I’ve lost a citation I made in an early draft. I based part of the reactions in my sketch on a line diagram which I earlier attributed (correctly or not) to the following reference,
Spiro, T.G., and Stigliani, W.M. (2003) Chemistry of the Environment. Upper Saddle River, NJ: Prentice Hall.