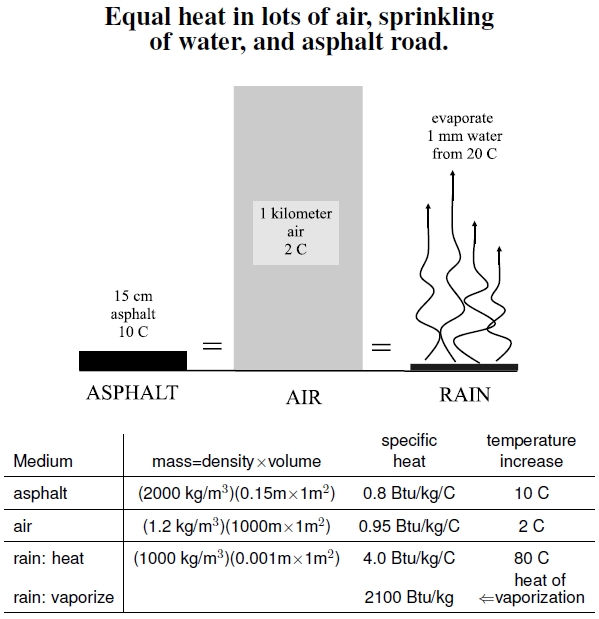
2.10 Heat Contents
Figure 2.10: Temperature changes involving equivalent heat contents in asphalt, air, and water. Suppose, in keeping with Figure 2.11, a 6-inch (0.15 m) thick square meter of parking lot asphalt heats up 10C during the day. If that heat were transferred uniformly to a 1 km tall air column above the parking lot, a 2C temperature change would result. After the parking lot heated up, a 1 mm, 20C rainfall could absorb all of that heat if it completely evaporated. The table provides all the numbers needed to do the math. Can transpiration of water by trees provide enough cooling to replace unreliable rainfalls?
Talk of urban heating and contemplating options for cooling means we need a basic discussion of temperature and heat energy. Though technical, a calculation of equivalent heat in each medium reveals important urban realities. Suppose we have a 6-inch (15 cm) thick road or sidewalk surface that heats up by some amount, say, 10C (see Figure 2.11). Further suppose that heat can go one of two places: either to increase the urban air temperature, or to heat up and evaporate water from an afternoon summer shower. Let’s compare the numbers.
The heat needed to change an object’s temperature depends on the product of three terms: its mass (1 gram, say, versus 1 kilogram), its “specific heat,” which roughly means its ability to hold heat (consider wood [low] versus concrete [high]), and, of course, the temperature change (1C versus 10C). Mathematically, we write this product for the heat involved in a temperature change ΔT (“delta tee”) as mcΔT, where the material considered has mass m and specific heat c.[33]
The table in Figure 2.10 summarizes the inputs into my calculations, and I provide them here for easy reference. The first three lines, asphalt, air, and rain:heat, compute heat content from raising temperatures. Multiplying density by volume — thickness times a meter-squared area—yields mass. Mass times specific heat times temperature change for each medium gives the required heat. In addition to heating rain to the boiling point, turning the water into vapor — the steam coming off a road during a hot day’s sprinkle of rain — requires more heat, noted as rain:vaporize. Multiplying water’s “heat of vaporization” by the mass of rain, then adding the result from the third row, gives the total heat needed to warm and evaporate a sprinkling of rain.
In the end, there’s roughly equal heat content in a 6-inch slab of concrete raised 10C, a kilometer high air column raised 2C (see Figure 2.7), and a 1 millimeter rainfall evaporated from 20C: 2,400 Btus vs. 2,280 Btus vs. 2,420 Btus.[34]
Notice that turning water at the boiling point into steam takes a lot more heat than getting the water to boil in the first place. Think about your personal experiences of heating water to the boiling point, taking relatively little time, but boiling all the water away takes much longer. That’s the difference between heating water and vaporizing water (read: turning it into steam). It’s my guess that during the summer thunderstorms dousing hot roadways, the first little bit of rain that falls quickly cools the pavement, in part, by evaporation, and the remaining rainfall has little cooling left to do, bringing temperatures down.
————————
[33]The densities of asphalt and concrete are about 2,200 and 2,400 kg/m3, respectively, and for simplicity I’ll assume 2,000 kg/m3 for both. For comparison, dry soil has a density of about 1,000 kg/m3. Asphalt, clay, and concrete specific heat values are about 0.19-0.22 cal/g/C, which means it takes about 0.2 calories of heat to increase the temperature of 1 gram of these materials by one degree Celsius. One British thermal unit (Btu) equals 252 calories and also equals 0.293 watt-hours. (One Btu is the amount of heat required to warm up one pound of water one degree Farhenheit at 60F.) For comparisons, burning a 100 Watt incandescent bulb for an hour uses 100 watt-hours of energy, about 341 Btu, or about 86,000 calories. That many calories would heat up a liter of water—1,000 grams, about a quart—by 86C, or from 14C, cool water, to 100C, which is the boiling point of water. In other words, burning a 100W incandescent bulb for an hour, or heating up water for pasta involves the same energy demand.
[34]I leave multiplying out the numbers in the table as an exercise for the reader! Another heat content calculation like the ones in Figure 2.10 shows that 2,400 Btu would heat up a 1-inch (2.54 cm) rainfall – about 25 liters of water spread over one square meter—from 20C up to a very warm temperature of 44C. In reality, of course, both the road and the water would equilibrate to some common temperature. That’s a lot of water needed to cool things down, but trees are cool because their transpiration makes a very important contribution to cooling, just as does the sprinkling of vaporized rain.